Chapter 4: Macronutrients
By Katelyn Barker, MS, RDN
Student contributor: Edgar Molin
Introduction
Culinary medicine aims to provide the knowledge and skills that support a nutritious eating plan that provides adequate energy and the nutrients essential for good health and function.
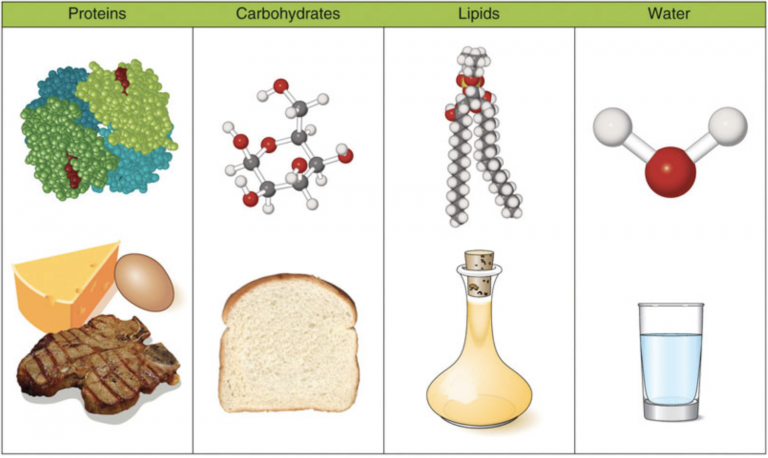
Nutrients are substances that are needed for growth, maintenance, and repair of body tissues. In the body, 3 nutrients provide energy: carbohydrates, protein, and fat. These energy-yielding nutrients are also referred to as macronutrients. The prefix makro is Greek for big or large, and because of their size, macronutrients account for most of the nutrients we consume in food.1,2
The body requires many grams of macronutrients per day, in contrast to micronutrients such as vitamins and minerals, which the body requires only in small amounts (milligrams or micrograms) per day.3 Water and fiber are also considered macronutrients. Although water provides no energy, and fiber provides (indirectly) only a small amount of energy, both are critical for optimal function and health. Consuming a variety of foods and beverages ensures adequate intakes of macronutrients. Having an awareness of the different nutrients each food provides can help culinary medicine practitioners and their clients understand the impact certain foods can have on health.
The energy released from carbohydrates, protein, and fat can be measured in calories, which are very small units of energy. Energy is expressed in 1,000-calorie metric units known as kilocalories (kcals), but these are commonly referred to as calories. When a food label identifies a slice of bread having 100 calories, this means 100 kcal.3
The amount of energy a food provides depends on the amount of carbohydrates, protein, and fat it contains. One gram of carbohydrate yields 4 kcal; 1 g of protein also yields 4 kcal; and 1 g of fat yields 9 kcal.
Carbohydrates
Carbohydrates are ideal nutrients to meet the body’s energy needs. Carbohydrates play a vital role in feeding the brain and nervous system and keeping the digestive system fit. It is important to understand that adequate carbohydrate intake throughout the day is essential to support the body’s energy needs. The body requires a minimum dietary intake of 130 g of carbohydrates to provide the brain an adequate amount of glucose.3
Role of Carbohydrates in the Body
During digestion, carbohydrates are broken down to glucose. Carbohydrates in the form of glucose provide energy to cells, tissues, and organs to carry out daily activities.4 Glucose is the brain and nervous system’s preferred source of energy and is essential to fuel its activities. Red blood cells use glucose exclusively.
Glycogen, the storage form of glucose, is used when the body needs glucose but isn’t getting it from food. The body obtains glucose and glycogen from consuming foods rich in carbohydrates. Carbohydrates can be used immediately for energy via glucose or converted into glycogen for reserve energy.1,3
Dietary Sources of Carbohydrates
The carbohydrate family includes sugars, starches, and fibers. Foods rich in carbohydrates include fruits, vegetables, grains, legumes, and milk. Nuts and seeds contain very little carbohydrate, and eggs, cheese, and fresh meat contain virtually no carbohydrates. Table 4.1 gives some examples of the carbohydrate content of foods.
Table 4.1. Examples of Carbohydrate Content in Foods4
|
Food |
Carbohydrate (approximate grams per serving) |
|---|---|
| Grains | |
| Bagel, 1 whole, 4 inches | 66 |
| Bread, wheat (1 slice) | 14 |
| Cereal, Cheerios (1 cup) | 21 |
| Oatmeal, instant (1 packet) | 28 |
| Crackers, club, multigrain (8 crackers) | 18 |
| Vegetables | |
| Potato, russet, baked (medium) | 37 |
| Carrots, raw (1 cup) | 12 |
| Corn (1 cup) | 27 |
| Tomatoes (one-half cup) | 15 |
| Fruit | |
| Apple, raw, with skin (medium) | 23 |
| Grapes, red or green, seedless (1 cup) | 27 |
| Banana (1 medium) | 27 |
| Raisins (1 oz) | 22 |
| Legumes | |
| Beans, black, boiled (one-half cup) | 20 |
| Lentils, green, boiled (one-half cup) | 20 |
| Dairy | |
| Milk, 2% fat (1 cup) | 12 |
| Yogurt, fruit variety (6 oz) | 32 |
| Yogurt, Greek, plain (6 oz) | 6 |
| Nuts | |
| Pecans (1 oz) | 4 |
| Almonds (1 oz) | <1 |
| Abbreviations: oz = ounce | |
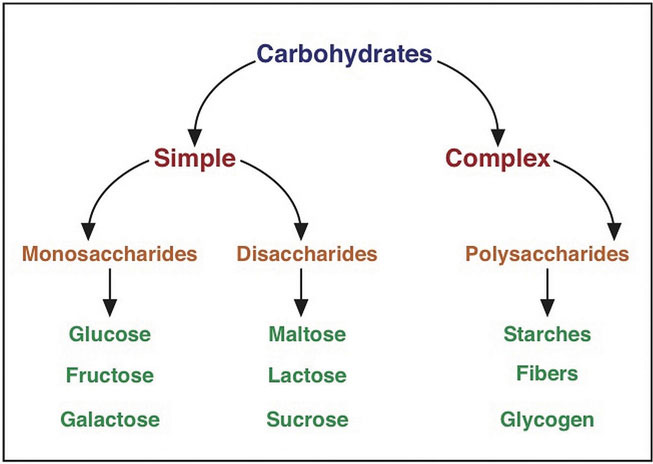
Classification of Carbohydrates

The dietary carbohydrate family includes:
- Monosaccharides: single sugars
- Disaccharides: pairs of monosaccharides
- Polysaccharides: chains of monosaccharides
Carbohydrates are broadly classified into 2 subgroups, based on their chemical composition and structure: simple and complex. Examples of simple carbohydrates or “simple sugars” are monosaccharides and disaccharides. Polysaccharides, which include starches and fibers, are usually considered complex carbohydrates.2,3 In culinary medicine, there are different scenarios in which simple and complex carbohydrates are beneficial for the body. Simple carbohydrates provide the body with quick energy and, therefore, may be beneficial for athletes engaging in high-intensity activities for which quick fuel is needed. Simple carbohydrates can also be used to correct low blood sugar in individuals who have hypoglycemia. Additionally, simple carbohydrates can be used in situations where quick nourishment is needed, such as when medical conditions affect the ability of a person to eat adequate amounts of foods. Complex carbohydrates, on the other hand, provide a steady source of energy and an increased amount of fiber. This makes these types of carbohydrates beneficial for digestive health, because many complex carbohydrates are high in fiber. Complex carbohydrates can also be beneficial for blood sugar control, because fiber helps to lower increases in blood sugar levels. This can be particularly helpful for people with diabetes or insulin resistance. Carbohydrates high in fiber can also help lower cholesterol and, therefore, reduce the risk of heart disease.3
Digestion and Absorption of Carbohydrates
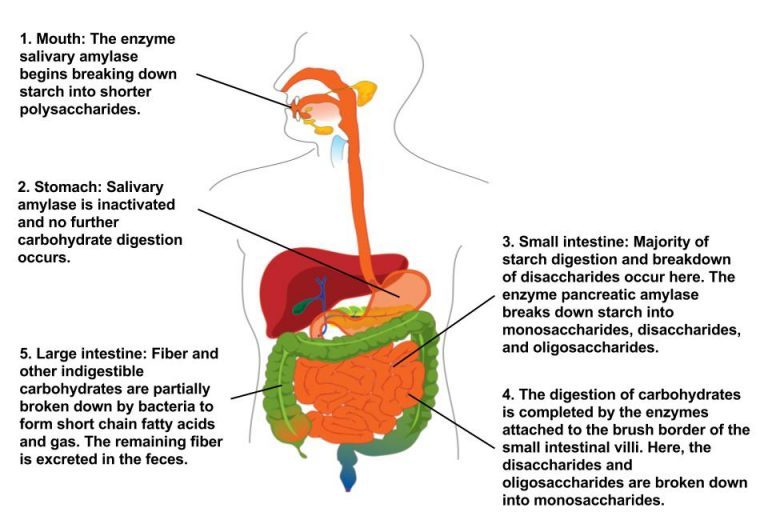
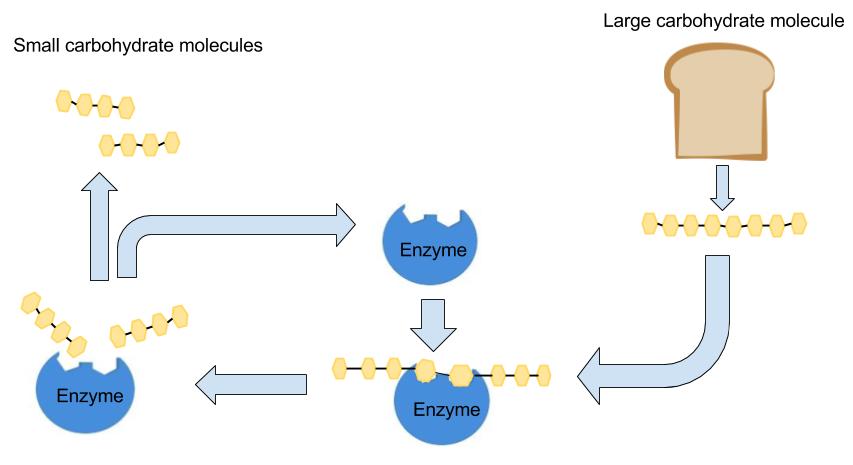
Digestion is necessary to break carbohydrates into smaller molecules that the body can more easily absorb and use. Large carbohydrate molecules such as starch require extensive breakdown, whereas smaller carbohydrate molecules such as monosaccharides and disaccharides are easier for the body to break down. Knowing how carbohydrates are digested can help us understand how different types of carbohydrates affect energy, blood sugar, digestive health, satiety, and overall health. Figure 4.4 provides a description of major organs and steps that are involved in digesting carbohydrates. These include the initial breakdown of starch in the mouth, further digestion in the stomach and small intestine, absorption in the small intestine, and partial digestion in the large intestine.
Carbohydrate digestion begins in the mouth when the salivary enzyme amylase starts to chemically breakdown starch to shorter polysaccharides. When carbohydrates reach the stomach, the activity of salivary amylase diminishes as the stomach’s acid and protein-digesting enzymes inactivate amylase. The stomach’s digestive juices contain no enzymes to break down carbohydrates. Fibers are not digested, but because they linger in the stomach, they delay gastric emptying, thereby increasing feelings of fullness and satiety. No further digestion of carbohydrate occurs until the small intestine.3
Most carbohydrate digestion takes place in the small intestine. When carbohydrates reach the small intestine, the pancreas produces pancreatic amylase, which enters the intestine via the pancreatic duct and continues to break down polysaccharides. The enzymes maltase, sucrase, and lactase continue to break down disaccharides into monosaccharides (mostly glucose).3 Within 1 to 4 hours, consumed sugars and starches are digested. Only the indigestible carbohydrates (e.g., fiber) remain in the digestive tract.
Dietary fiber cannot be broken down by digestive enzymes. As dietary fiber lingers in the large intestine, it attracts water, which softens the stool for passage out of the body. Bacteria in the gastrointestinal tract ferment some of the fiber. This process generates water, gas, and short-chain fatty acids.3
Lipids
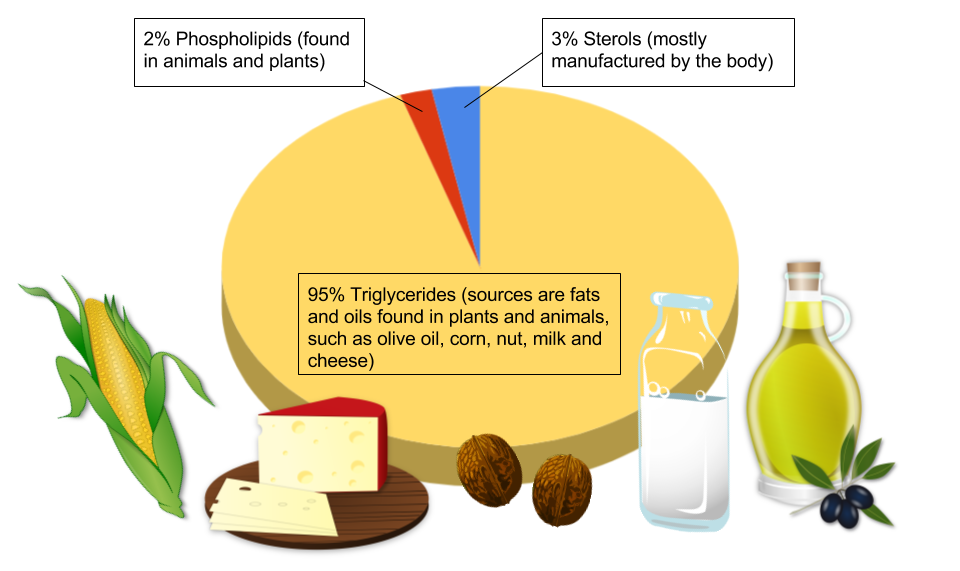
Lipids are important molecules that serve different roles in the human body. Fat is a type of lipid, but the 2 terms are often used interchangeably. The lipid family includes triglycerides, phospholipids, and sterols, and these lipids are primarily consumed through fats and oils. It is important to understand the impact fats and lipids have on the body and in cooking, as well as on the flavor and texture of foods.
Role of Lipids in the Body
Lipids provide most of the energy needed to perform the body’s work. They are also the chief storage form of the energy we derive from food when we consume more than needed. The storage of fat is an important survival mechanism: stored fat helps keep people alive during times of famine. Compared with carbohydrates, fat is more easily stored because it can pack tightly together without water and can store much more energy in a small space. Fat provides more than twice the energy of carbohydrate or protein, making it the most efficient storage form of energy.1

Fat serves many other purposes in the body too. Pads of fat surrounding the vital organs are protective and serve as shock absorbers. Fat plays a critical role in internal climate control by insulating the body and slowing heat loss in cold temperatures. Lipids also serve as raw material for making several products needed by the body, including vitamin D, which is essential for bone health; bile, which assists in digestion; and lipid hormones, which regulate tissue functions.1
Lipids are needed for absorption of some essential nutrients, and some amount of fat in the diet is necessary for their absorption. These nutrients are fat-soluble vitamins: A, D, E, and K; and essential fatty acids.1
Fat carries many sensory qualities that enhance the aroma, texture, and flavor of food. Fat lends crispness to fried foods and tenderness to foods such as meats and baked goods. Fat also contributes to satiety, which is the satisfaction of feeling full after a meal. Foods that contain fat trigger physiological events that suppress the desire to eat.1
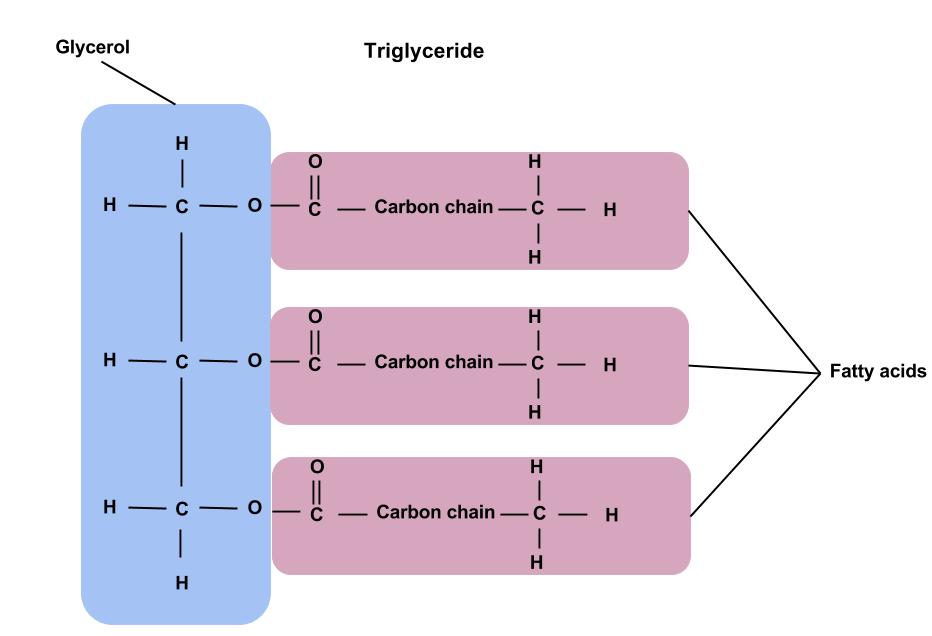
Classification of Lipids
Triglycerides make up most of the lipid present in the body and in food. Triglycerides are composed of 3 (tri) fatty acids that are attached to a molecule of glycerol (hence, triglyceride). Fatty acids can differ from one another in 2 different ways: the chain length and the degree of chemical bond saturation. Triglycerides usually include a mixture of various fatty acids, and the mixture determines whether a fat will be harder or softer at room temperature.1 Understanding how the structure of lipids affects each type of fat can helps us choose fats that are beneficial to our health.
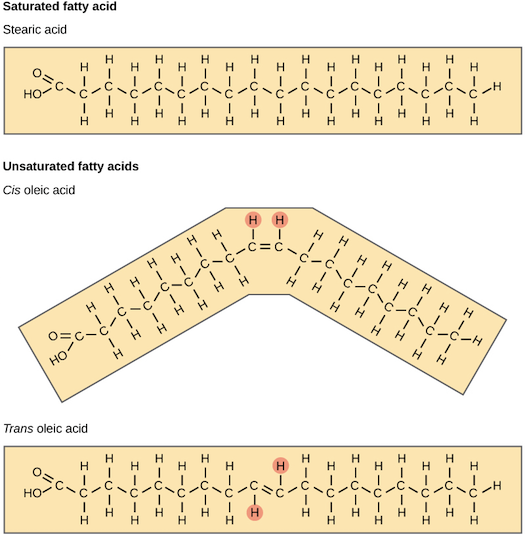
Saturation refers to whether a fatty acid chain contains all the hydrogen atoms its structure allows. If every available carbon bond is holding a hydrogen atom, the chain is a saturated fatty acid. If the carbon bond has 1 or more points of unsaturation, or a place where hydrogens are missing, the fatty acid chain is an unsaturated fatty acid.
With 1 point of unsaturation, the fatty acid is called a monounsaturated fatty acid. With 2 or more points of unsaturation, the fatty acid is called a polyunsaturated fatty acid. Often, a single triglyceride contains both saturated and unsaturated fatty acids of varying length, making it a mixed triglyceride.1
The degree of saturation of the fatty acids affects the temperature at which it melts. Generally, the more unsaturated fatty acids present in a fat, the more liquid the fat will be at room temperature. The more saturated fatty acids are present, the more solid the fat will be at room temperature.1
The presence of a double bond in a fatty acid can result in different structures. When the hydrogen atoms are bonded to the same side of the carbon chain, this is called a cis fatty acid. Because the hydrogen atoms are on the same side, the fatty acid has a bent structure. Naturally occurring fatty acids usually have a cis configuration. In a trans configured fatty acid, the hydrogen atoms are attached on opposite sides of the carbon chain. Trans fats are not usually found naturally in foods but are the product of a process called hydrogenation. Hydrogenation adds hydrogen to the double bonds, thus making the fatty acid saturated. This is how vegetable oils are converted into semisolid fats for the use of manufacturing process.2 When reading ingredients on a nutrition label, it can be helpful to understand that the term hydrogenated means a food contains trans fats. Foods that contain trans fats will include hydrogenated or partially hydrogenated items in the ingredient list on a food label.
The last 2 types of lipids, phospholipids and sterols, are present in lower quantities in food and in the body. These types of lipids play important structural and regulatory roles in the body. Phospholipids bind together in a strong double layer that forms the membranes of cells and they generate signals inside cells in response to hormones, such as insulin, to help control bodily conditions. Sterols, such as cholesterol, are also important in the structure of the cell membrane. Cholesterol also serves as the raw material for making emulsifiers in bile, which is important to fat digestion.
Essential Fatty Acids
The body is capable of synthesizing most of the fatty acids it needs from food, and these fatty acids are known as nonessential fatty acids. However, there are some fatty acids that the body cannot synthesize, and these are called essential fatty acids. Essential fatty acids must be obtained from food.1,2
There are 2 categories of essential fatty acids: omega-3 and omega-6. The 3 and the 6 refer to the position of the first carbon double bond. Omega-6 fatty acids are precursors to important compounds called eicosanoids. Eicosanoids act somewhat like hormones by helping to regulate body functions. Omega-3 fatty acids are precursors to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which form their own eicosanoids that often oppose those from omega-6. For example, omega-3 eicosanoid relaxes blood vessels and lowers blood pressure, whereas an omega-6 eicosanoid constricts the vessels and increases blood pressure. A balance between the 2, therefore, promotes normal blood pressure.1,2
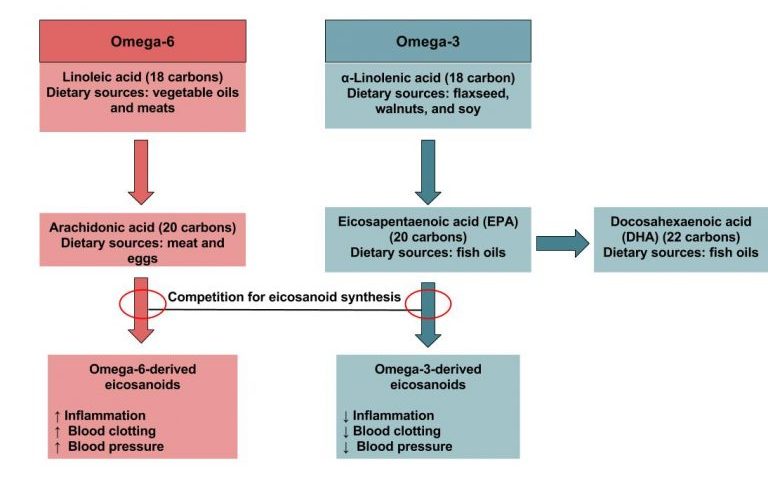
Knowing foods that are a good source of omega-3 and omega-6 fatty acids can enable us to incorporate these foods into daily intake. Omega-3 and omega-6 fatty acids are both a type of unsaturated fat and are found in foods such as fish, flaxseed oil, hemp, walnuts, and leafy vegetables. Table 4.2 shows food sources of omega-6 and omega-3 fatty acids. In the United States and Canada, deficiencies of omega-3 and omega-6 are almost unknown in otherwise healthy adults.1,2
Table 4.2. Food Sources of Omega-6 and Omega-3 Fatty Acids1
| Fatty acid | Food source |
|---|---|
|
Omega-6 |
Nuts and seeds (e.g., cashews, walnuts, sunflower seeds) Poultry fat Vegetable oils (e.g., corn, safflower, sesame, soybean, sunflower) |
| Omega-3 |
Nuts and seeds (e.g., chia seeds, flaxseeds, walnuts, soybeans) Vegetable oils (e.g., canola, flaxseed, soybean, walnut, wheat germ) Fish and seafood |
Dietary Sources of Lipids
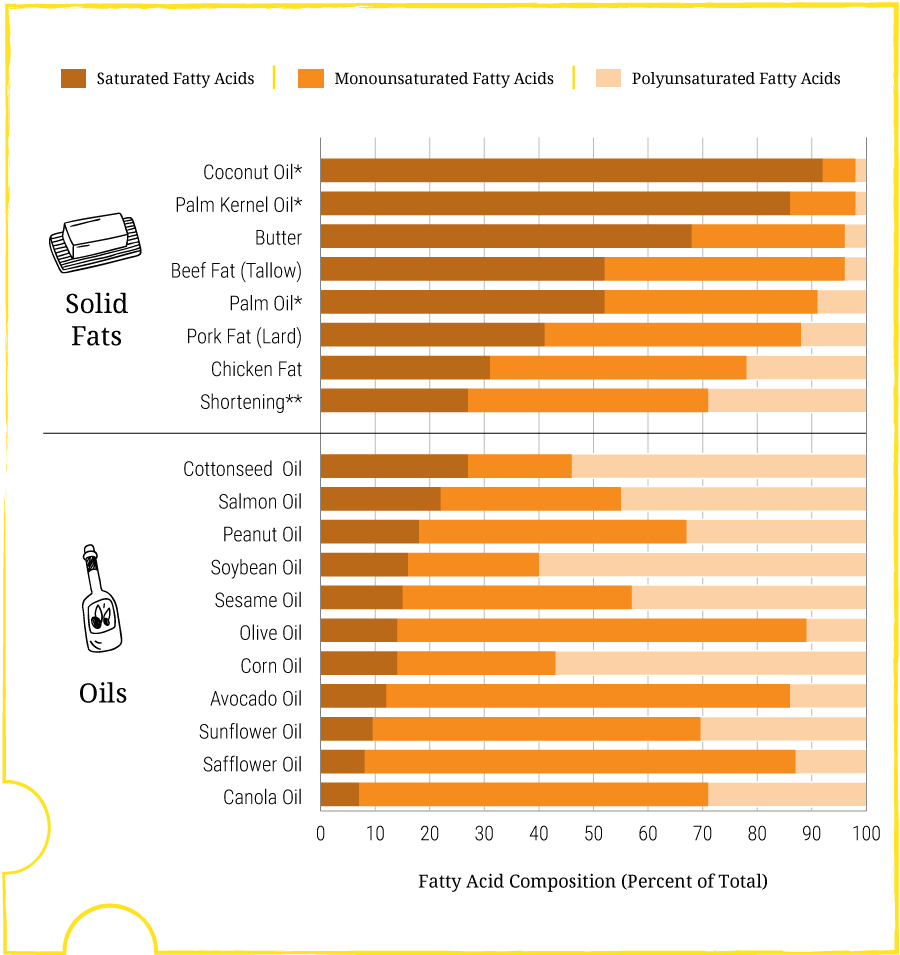
All foods contain a combination of saturated and unsaturated fats. Some foods contain higher ratios of saturated or unsaturated fats, as shown in Figure 4.10. Animal-based fats generally contain the most saturated fat. However, some oils, such as coconut oil and palm oil, are primarily made of saturated fat. Other vegetable oils and fish oils are rich in polyunsaturated fatty acids. Some vegetable oils are also rich in monounsaturated fatty acids. Figure 4.10 shows how dietary fats contain a mixture of saturated, monounsaturated, and polyunsaturated fatty acids.
Saturated fats are found in animal products (e.g., butter, cheese, meats) and some plant oils (e.g., coconut oil, palm oil). Increased intake of saturated fats is associated with increased blood levels of cholesterol and blood clotting, which can increase the risk for heart disease. The US Dietary Guidelines suggest aiming for less than 10% of daily energy intake from saturated fats. Whereas trans fats may be implicated in chronic disease such as heart disease, unsaturated fats seem to offer health benefits. For this reason, dietary recommendations suggest replacing sources of saturated fats and trans fats with foods rich in unsaturated fats. Unsaturated fats are found in seafood, nuts, seeds, and vegetable oils.
** Shortening may be made from partially hydrogenated vegetable oil, which contains trans fatty acids.
Digestion and Absorption of Lipids
Lipid digestion begins in the mouth, with some hard fats melting as they reach body temperature. When fat-containing food reaches the stomach, the strong muscle contractions of the stomach propel the partially digested food back and forth. The churning of the stomach grinds the solid pieces into finer particles and disperses fat into smaller droplets. These actions help expose the fat for attack by the gastric enzyme lipase.
Most fat digestion takes place in the small intestine.3 When fat enters the small intestine, it triggers the release of bile by the gallbladder. Bile acts as an emulsifier that facilitates the mixing of fat molecules in the surrounding watery fluids. Bile is not a digestive enzyme, but it is essential in preparing fat for digestion by the lipase enzymes from the pancreas and small intestine.3
The major fat-digesting enzymes are pancreatic lipase and some intestinal lipases. These enzymes remove each triglyceride’s fatty acids. These fatty acids and the remaining fragments of the triglyceride are absorbed by the intestinal cells directly into the bloodstream, where they are packaged and transported to tissues in the body where they are needed.3 Understanding how lipids are digested and used in the body helps us, in turn, to understand the role lipids play in nutrient absorption, energy utilization, and health conditions.
Protein

Protein performs many vital functions in the body. Proteins are made up of amino acids, and each protein contains a different combination of amino acids. It is important to understand the key role protein plays in essential functions of the body, balancing the absorption of carbohydrates and promoting satiety. For healthy adults, 0.8 g of protein per kilogram of body weight is recommended.3
Amino Acids
Each amino acid has a distinctive side chain attached to the center carbon of the molecule. This side chain gives each amino acid its identity and chemical nature. There are 20 amino acids, each with a different side chain, and these make up most of the proteins of living tissues. A typical protein is made up of 300 or more amino acids, and the specific sequence of amino acids are unique to each protein. Depending on the number and sequence of amino acids, the resulting protein will fold into a specific shape. This shape determines the protein’s function (e.g., muscle or enzyme).1,5
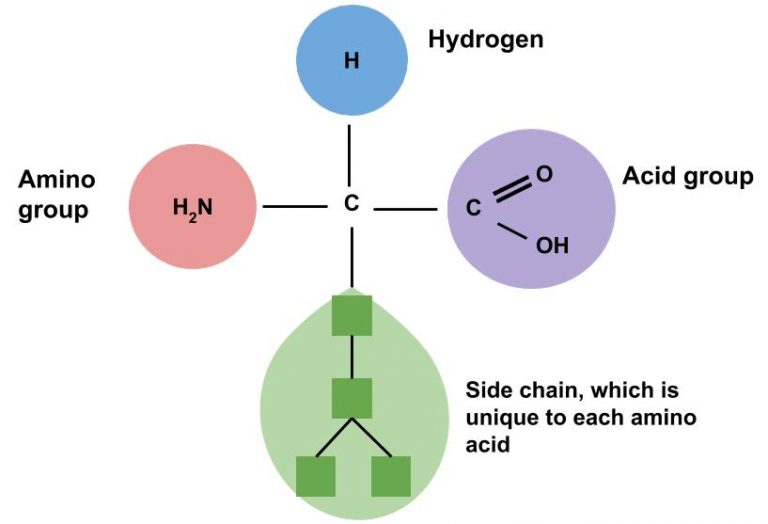
Amino acids are classified as essential or nonessential. The body can produce more than half of the 20 amino acids for itself (given it has all the parts needed), and these are known as nonessential amino acids. There are 9 amino acids that the body cannot manufacture, or that it manufactures too slowly to meet its needs. These are known as essential amino acids and must be obtained from food.1,3,5 Sometimes during infancy, growth, and in diseased states, the body cannot synthesize enough of some of the nonessential amino acids and more of them are required in the diet. These types of amino acids are called conditionally essential amino acids. Essential and nonessential amino acids are summarized in Table 4.3.6
Table 4.3. Essential and Nonessential Amino Acids6
|
Essential |
Nonessential |
|---|---|
|
Histidine Isoleucine Leucine Lysine Methionine Phenylalanine Threonine Tryptophan Valine |
Alanine Arginine* Asparagine Aspartic acid Cysteine* Glutamic acid Glutamine Glycine* Proline* Serine Tyrosine* |
|
* This amino acid is conditionally essential. It must be obtained in the diet in certain situations when more are needed than the body can synthesize. |
|
Role of Protein in the Body
Amino acids must be continuously available to build the proteins of new tissue. Proteins regulate gene expression; serve as enzymes, hormones, and antibodies; transport substances throughout the body; maintain fluid and electrolyte balance and acid-base balance; provide structure in tendons, ligaments, and scars; and play a vital role in wound healing and tissue regeneration.1,2
One of the most important functions of protein is as an enzyme. Enzymes are proteins that catalyze a specific chemical reaction. Thousands of enzymes are found inside a single cell, and each facilitates a specific chemical reaction.1,2
The body’s hormones are messenger molecules, and many of them comprise amino acids. Many glands in the human body produce hormones. Among these are the endocrine glands, and when the endocrine glands are stimulated, they release a hormone. The hormone is then transported in the blood to its target cell, where it communicates a message to initiate a specific reaction or cellular process. For example, in response to eating a meal, blood glucose levels begin to increase. In response to increased blood glucose levels, the pancreas releases the hormone insulin. The presence of insulin signals the cells to take up glucose and use it for energy or store it for use later.2
Protein is also essential in maintaining the proper pH balance of the blood. The body has several mechanisms that hold the blood pH within normal range. One of these mechanisms involves circulating albumin, a slightly acidic protein that acts as a buffer against abrupt changes in the concentration of positively charged molecules (e.g., proteins, calcium, potassium, magnesium).2
Albumin and another important protein, hemoglobin, also play vital roles in molecular transport. Albumin chemically binds to hormones, fatty acids, some vitamins, essential minerals, and drugs, and transports them throughout the circulatory system. Red blood cells, of which hemoglobin is a part, bind oxygen in the lungs and transport oxygen to all tissues in the body.2
Dietary Sources of Protein
The protein food group consists of foods made from meat, seafood, poultry, eggs, soy, dry beans, peas, and seeds. Protein sources differ in their additional components. Protein-rich animal-based foods commonly have high amounts of B vitamins, vitamin E, iron, magnesium, and zinc. Some animal-based protein-rich foods have an increased amount of saturated fat and cholesterol.
Plant-based protein-rich foods provide fiber, contain less saturated fat, and are more environmentally sustainable in comparison with animal-based proteins. Plant-based protein-rich foods include beans, peas, lentils, nuts, seeds, soy, and fortified soy beverages.2 Table 4.4 lists the protein content in some common foods.
Table 4.4. Protein in Common Foods6
| Animal source | Grams of protein per standard serving |
|---|---|
| Egg white | 3 g per 1 large white |
| Whole egg | 6 g per 1 large egg |
| Cheddar cheese | 7 g/oz (30 g) |
| Milk, 1% fat | 8 g per 1 cup (8 fl oz) |
| Yogurt | 11 g per 8 oz |
| Greek yogurt | 22 g per 8 oz |
| Cottage cheese | 15 g per ½ cup |
| Hamburger | 30 g per 4 oz |
| Chicken | 35 g per 4 oz |
| Tuna | 40 g per 6 oz can |
| Plant source | Grams of protein per standard serving |
| Almonds, dried | 6 g per 1 oz |
| Almond milk | 1 g per cup (8 fl oz) |
| Soy milk | 8 g per cup (8 fl oz) |
| Peanut butter | 4 g per 1 tbsp |
| Hummus | 8 g per ½ cup |
| Refried beans | 6 g per ½ cup |
| Lentil soup | 11 g per 10.5 oz |
| Tofu, extra firm | 11 g per 3.5 oz |
| Enriched wheat bread | 1 g per slice (45 g) |
| Whole-grain bread | 5 g per slice (45 g) |
| Grape Nuts cereal | 7 g per ½ cup |
| Abbreviations: g = gram; oz = ounce; tbsp = tablespoon; fl oz = fluid ounce. | |
Protein Quality

There are 2 factors that influence protein quality: the protein’s digestibility and its amino acid composition. High-quality proteins provide enough of all the essential amino acids needed to support the body’s work. In the United States and other countries where nutritious foods are abundant, most people eat protein in large enough quantities that they receive all the amino acids they need. In countries where food is scarce and people eat only marginal amounts of protein-rich foods, the quality of the protein becomes critical.3
Protein can be found in both plant- and animal-based foods. In general, plant proteins are of lower quality than animal proteins because they usually don’t provide all the essential amino acids in 1 source. For this reason, protein with different but complementary amino acids can be combined to provide all the essential amino acids needed to support health. It is not necessary to balance amino acids at each meal if protein intake is varied and energy intake is sufficient.1 Table 4.5 lists sources of complementary proteins.
Table 4.5. Complementary Protein Sources6
| Food | Lacking amino acids | Complementary food | Complementary menu |
|---|---|---|---|
| Legumes | Methionine, tryptophan | Grains, nuts, and seeds | Hummus and whole-wheat pita |
| Grains | Lysine, isoleucine, threonine | Legumes | Cornbread and kidney bean chili |
| Nuts and seeds | Lysine, isoleucine | Legumes | Stir-fried tofu with cashews |
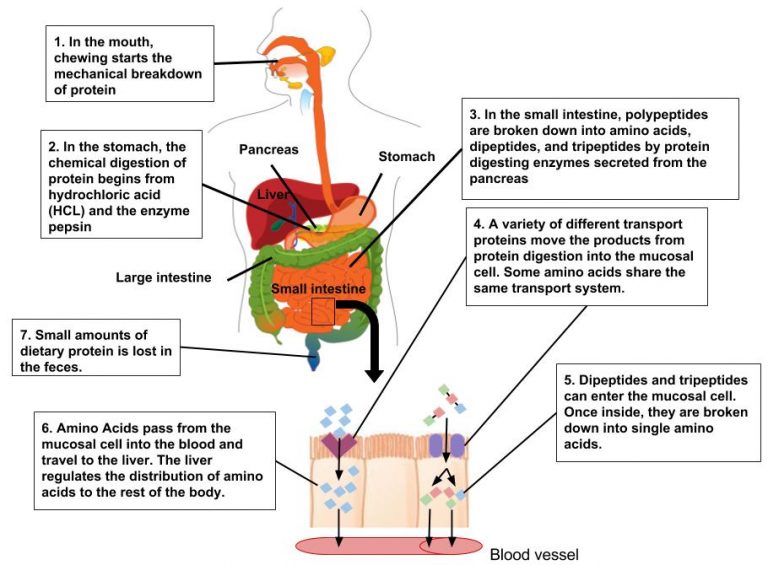
Digestion and Absorption of Protein
Proteins in foods do not directly become proteins in the body. Instead, dietary protein provides amino acids from which the body manufactures its own proteins.
Protein digestion begins in the stomach, where hydrochloric acid uncoils protein strands so digestive enzymes can attack the peptide bonds. Pepsin, a gastric enzyme that hydrolyzes protein, cleaves proteins into small polypeptides and amino acids. When polypeptides then enter the small intestine, several pancreatic and intestinal proteases hydrolyze them further into short peptide chains and amino acids. Then, peptidase enzymes split most of the dipeptides and tripeptides into single amino acids. These single amino acids are then absorbed by the intestinal cells, where they can be used for energy or to synthesize needed compounds.3
Key Takeaways
- Carbohydrates, protein, and fat are energy-yielding nutrients that provide the body materials needed for growth, maintenance, and repair of body tissues.
- The amount of energy (or calories) a food provides depends on the amount of carbohydrates, protein, and fat it contains.
- Carbohydrate’s major function is to provide energy for the body.
- Simple carbohydrates provide the body with a quick energy source, whereas complex carbohydrates provide additional fiber and blood sugar control, and take longer to metabolize.
- Foods rich in carbohydrates include fruits, vegetables, grains, legumes, and milk. Nuts and seeds contain very little carbohydrates, and eggs, cheese, and fresh meat contain virtually no carbohydrates.
- Lipids provide most of the energy needed to perform the body’s work and are the primary storage form for the energy from food.
- Saturated and trans fatty acids generally increase the risk for heart disease, whereas unsaturated fatty acids are health promoting.
- Essential fatty acids must be obtained from food and fall into 2 categories: omega-3 and omega-6. Omega-3 and omega-6 fatty acids are beneficial for health, especially heart health.
- Proteins are made up of amino acids, and each protein contains a different combination of amino acids.
- Protein can be found in both plant- and animal-based foods. Balancing amino acids at each meal is unnecessary if protein intake is varied and energy intake is sufficient.
References
- Sizer FS, Whitney EN. Nutrition Concepts and Controversies. Cengage Learning; 2020.
- Titchenal A, Hara S, Caacbay NA, et al. Human Nutrition: 2020 Edition. University of Hawai’i at Mānoa Food Science and Human Nutrition Program. OER Commons; 2020. Accessed April 2, 2023. https://pressbooks.oer.hawaii.edu/humannutrition2/
- Rolfes SR, Pinna K, Whitney E. Understanding Normal and Clinical Nutrition. Cengage Learning; 2021.
- Gal NJ, Ford AL, Dahl WJ. Facts about carbohydrate. Publication no. FSHN14-03/FS243. July 18, 2021. Accessed April 22, 2023. https://edis.ifas.ufl.edu/publication/FS243
- What are proteins and what is their function in the body? European Food Information Council. December 16, 2019. Accessed May 15, 2023. https://www.eufic.org/en/whats-in-food/article/what-are-proteins-and-what-is-their-function-in-the-body.
- Callahan A, Leonard H, Powell T. Nutrition: Science and Everyday Application. Open Oregon Educational Resources. OER Commons; 2020. Accessed August 18, 2023. https://openoregon.pressbooks.pub/nutritionscience2e/
Compounds composed of single or multiple sugars. Source: Nutrition Concepts and Controversies, 15th Edition
The nutrients the body can use for energy: carbohydrate, protein, and fat. Source: Nutrition Concepts and Controversies, 15th Edition
Nutrients your body needs in large amounts to function optimally, such as carbohydrates, protein, and fat. Source: WebMD
The unit used to measure the energy in foods is a kilocalorie; it is the amount of heat energy necessary to raise the temperature of a kilogram (a liter) of water 1 degree Celsius. Source: Nutrition Concepts and Controversies, 15th Edition
A single sugar used in plant and animal tissues for energy. Source: Nutrition Concepts and Controversies, 15th Edition
A highly branched polysaccharide that is made and held in liver and muscle tissues as a storage form of glucose. Source: Nutrition Concepts and Controversies, 15th Edition
Simple carbohydrates; that is, molecules of either single sugar units or pairs of those sugar units bonded together. Source: Nutrition Concepts and Controversies, 15th Edition
Plant polysaccharides composed of glucose. Source: Nutrition Concepts and Controversies, 15th Edition
The indigestible parts of plant foods. Source: Nutrition Concepts and Controversies, 15th Edition
Sugars, including both single sugar units and linked pairs of sugar units. Source: Nutrition Concepts and Controversies, 15th Edition
Long chains of sugar units arranged to form starch or fiber; also called polysaccharides. Source: Nutrition Concepts and Controversies, 15th Edition
A hormone secreted by special cells in the pancreas in response to elevated blood glucose concentration. Insulin controls the transport of glucose from the bloodstream into the muscle and fat cells. Source: Understanding Normal and Clinical Nutrition, 12th Edition
An enzyme that breaks down amylose (a form of starch). Source: Understanding Normal and Clinical Nutrition, 12th Edition
The feeling of fullness and satisfaction that occurs after a meal. Source: Understanding Normal and Clinical Nutrition, 12th Edition
One of 3 main classes of dietary lipids and the chief form of fat in foods and the human body. A triglyceride is made up of 3 units of fatty acids and 1 unit of glycerol. Source: Nutrition Concepts and Controversies, 15th Edition
One of 3 main classes of dietary lipids. These lipids are present in all cell membranes. Source: Nutrition Concepts and Controversies, 15th Edition
One of the 3 main classes of dietary lipids. Sterols have a structure similar to that of cholesterol. Source: Nutrition Concepts and Controversies, 15th Edition
A group of compounds composed of oxygen, hydrogen, and carbon atoms that supply the body with energy. Source: Merriam-Webster Online Dictionary
Lipids that are liquid at room temperature. Source: Nutrition Concepts and Controversies, 15th Edition
An emulsifier made by the liver from cholesterol, stored in the gallbladder, and released into the small intestine when needed. Bile does not digest fat but emulsifies it so that enzymes may contact it and begin digesting fatty acids. Source: Nutrition Concepts and Controversies, 15th Edition
Required by humans and other animals for normal physiological function that cannot be synthesized by the body. Source: Wikipedia
Carries the maximum possible number of hydrogen atoms (having no points of unsaturation). Source: Nutrition Concepts and Controversies, 15th Edition
Lacks some hydrogen atoms and has 1 or more points of unsaturation. Source: Nutrition Concepts and Controversies, 15th Edition
Contains 1 point of unsaturation. Source: Nutrition Concepts and Controversies, 15th Edition
Contains 2 or more points of unsaturation. Source: Nutrition Concepts and Controversies, 15th Edition
A polyunsaturated fatty acid with its endmost double bond 3 carbons from the end of the carbon chain. Linolenic acid is an example. Source: Nutrition Concepts and Controversies, 15th Edition
A polyunsaturated fatty acid with its endmost double bond six carbons from the end of the carbon chain. Linoleic acid is an example. Source: Nutrition Concepts and Controversies, 15th Edition
Biologically active compounds that regulate body functions. Source: Nutrition Concepts and Controversies, 15th Edition
Eicosatetraenoic acid (EPA) and docosahexaenoic acid (DHA); omega-3 fatty acids made from linolenic acid in the tissues of fish. Source: Nutrition Concepts and Controversies, 15th Edition
Fats found in animal-based foods such as beef, pork, poultry, full-fat dairy products, eggs, and tropical oils. Because they are typically solid at room temperature, they are sometimes called “solid fats.” Source: American Heart Association
Enzymes that hydrolyze lipids. Gastric lipase is a fat-digesting enzyme secreted from the cells of the stomach. Pancreatic lipase is a fat-digesting enzyme secreted from the pancreas. Source: Understanding Normal and Clinical Nutrition, 12th Edition
A substance with both water-soluble and fat-soluble portions that promotes the mixing of oils and fats in a watery solution. Source: Understanding Normal and Clinical Nutrition, 12th Edition
The building blocks of protein. Each has an amine group at 1 end, an acid group at the other, and a distinctive side chain. Source: Nutrition Concepts and Controversies, 15th Edition
Amino acids the body can make. Source: Understanding Normal and Clinical Nutrition, 12th Edition
Amino acids the body cannot make, so must be obtained from the diet. Source: Understanding Normal and Clinical Nutrition, 12th Edition
Amino acids that must be obtained in the diet in certain situations when more are needed than the body can synthesize. Source: Nutrition Science and Everyday Application, 2nd Edition
Proteins that facilitate chemical reactions without being changed in the process; protein catalysts. Source: Understanding Normal and Clinical Nutrition, 12th Edition
Chemical messengers secreted by a variety of glands in response to altered conditions in the body that travel to 1 or more specific target tissues or organs, where it elicits a specific response to maintain homeostasis. Source: Understanding Normal and Clinical Nutrition, 12th Edition
Water-soluble proteins that occur in blood plasma or serum, muscle, the whites of eggs, milk, and other animal substances, and in plant tissues and fluids. Source: Merriam-Webster Online Dictionary
Any numerous iron-containing respiratory pigments of various organisms. Source: Merriam-Webster Online Dictionary
A gastric enzyme that hydrolyzes protein. Pepsin is secreted in an inactive form, pepsinogen, which is activated by hydrochloric acid in the stomach. Source: Understanding Normal and Clinical Nutrition, 12th Edition
Undergoes the process of hydrolysis (a chemical process of decomposition involving the splitting of a bond and the addition of the hydrogen cation and the hydroxide anion of water). Source: Merriam-Webster Online Dictionary
Enzymes that hydrolyze protein. Source: Understanding Normal and Clinical Nutrition, 12th Edition
A digestive enzyme that hydrolyzes peptide bonds. Tripeptidases cleave tripeptides; dipeptidases cleave dipeptides. Source: Understanding Normal and Clinical Nutrition, 12th Edition

